The Tahara research group covers pharmaceutical formulation design, DDS, and manufacturing process intensification with respect to particle/fiber engineering and powder technology.

Research on non-invasive DDS
using nano-drug carriers
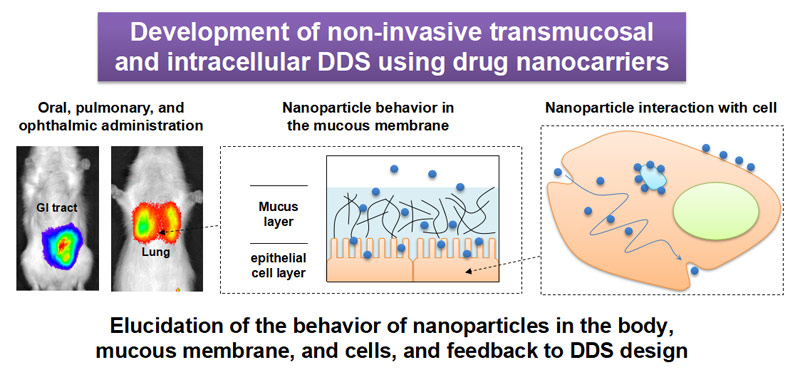
Biocompatible nanoparticles, such as liposomes and polymeric particles have relatively large specific surface areas which improve interactions with tissues and cells. They have properties which help particles enter mucus layers, although larger microparticles are limited in their uptake by the mucin matrix.
We are interested in the unique interactions of nanoparticles with mucous membranes and cells, and we focus on the development of non-invasive patient-friendly DDS systems. We also believe topical administration is an effective way to maximize the characteristics of nano-particulate carriers. For local administration, where a drug is administered directly at the disease site, drug targeting is primarily achieved at administration, thus transition to other organs/tissues is minimized.
Nanoparticles target gastric mucosa by oral administration to improve systemic drug absorption, and to treat inflammatory bowel diseases. Similarly, targeting lung mucosa via pulmonary nanoparticle administration has improved the systemic absorption of drugs, and maximized the pharmacological effects for topical lung diseases, including asthma and chronic obstructive pulmonary disease. We are also developing an ocular DDS which uses nanoparticles for topical administration; it targets the eye mucus around the palpebral conjunctiva to deliver nanoparticles/therapeutics to eye anterior/posterior segments.

Particle/formulation design for
improved drug bioavailability
We are developing drug nanocrystal preparations, solid dispersions, and next-generation nanofiber preparations which improve the solubility of poorly water-soluble drugs. We are also developing patient-friendly formulations such as orally disintegrating (OD) tablets, OD films, and mucosal adhesive preparations which are easy to take, even for children and patients who have difficulty swallowing.


Development of an integrated
pharmaceutical continuous
production system
We are developing an end-to-end integrated pharmaceutical manufacturing process, from drug synthesis to final formulation. In particular, we are developing novel pharmaceutical manufacturing technologies, and focusing on spherical crystallization and continuous formulation (powder) processes which integrate purification and granulation processes. Data incorporating multivariate analysis and machine learning are being used to analyze our manufacturing processes. These developments will optimize manufacturing processes, and are important research interests.


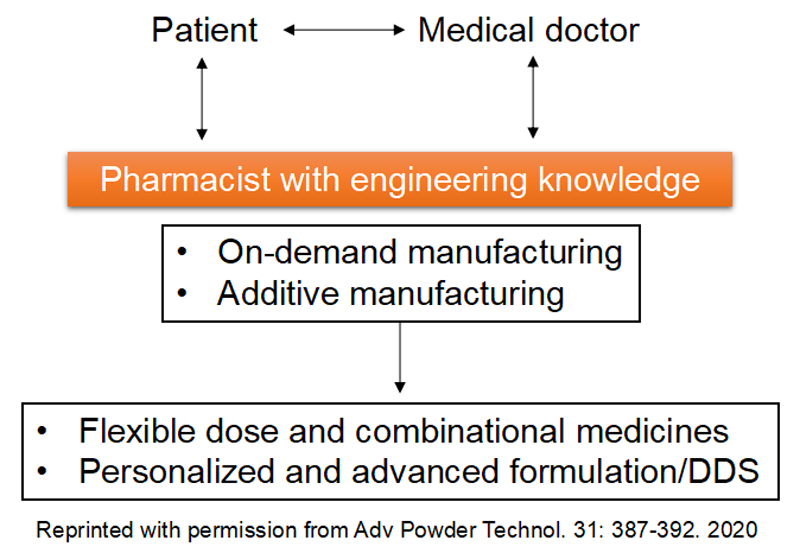
Research on personalized
pharmaceutical formulations
New approaches to pharmaceutical manufacturing include on-demand manufacturing technology in a box and 3D printing. Such advancements could facilitate point-of-care and pharmacy-based pharmaceutical production, which may be the future of pharmaceuticals for advanced pharmacy compounding and hospital formulations. These methods are used by pharmacists when doctors cannot use commercial pharmaceuticals to provide medical therapies suitable for patients. These new technologies will underpin future pharmaceutical manufacturing, and be tailored to personalized medicines and small-patient groups, to provide flexible dose/combinational drugs, orphan drugs, and pediatric formulations, which cannot be generated by conventional mass production. On-demand pharmaceutical products can also be derived to produce personalized formulations in accordance with specific, individualized prescriptions, thereby, revolutionizing pharmacy compounding.
We are interested in on-demand pharmaceutical manufacturing technologies, including quality control of in-hospital preparations and tablet molding using 3D printers, which facilitate future personalized formulations.